- Admissions
- Academics
- Research Office
- Student Life
- News & Events
- Outreach
- About
Dr. M. Farooq Rai is an Assistant Professor at Khalifa University College of Medicine and Health Sciences in the Department of Biological Sciences. He was a research faculty at Washington University School of Medicine in USA prior to joining Khalifa University. Dr. Rai obtained his PhD in Biochemistry from the Free University of Berlin and has been trained in cellular and molecular biology with extensive experience in generating intelligent constructs to regulate the expression of therapeutic gene(s) in osteoarthritis. He has 15 years of experience in developing and studying genetic mouse models of cartilage repair and osteoarthritis and defining early osteoarthritis in patients using RNA screening (transcriptomic) techniques to define phenotypes of cartilage, ligament and meniscus after knee injury. Dr. Rai’s research program is focused on identifying early molecular events in the process of osteoarthritis with the goal of developing novel treatment options to arrest these early molecular manifestations and prevent late sequelae of the disease. He received several research awards and fellowships from various foundations and the National Institutes of Health: German/Pakistan PhD Fellowship; T32 Ruth L. Kirschstein Postdoctoral Fellowship; Arthritis Research Award; and K99/R00 Pathway to Independence Award. Dr. Rai has presented at numerous national and international conferences and has won a number of travel fellowships and other prestigious awards including New Investigator Recognition Award (twice) and Alice L Jee Award from the Orthopedic Research Society, Cabaud Memorial Award (twice) from the American Orthopaedic Society for Sports Medicine, Early Career Award from the Journal of Orthopedic Research, and Harold M. Frost Young Investigator Award from the American Society for Bone and Mineral Research.
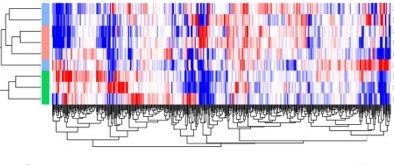
Early molecular detection of post-traumatic osteoarthritis
My laboratory investigates the basic and translational aspects of musculoskeletal research. The focus is on tissue injury, repair and degeneration (osteoarthritis). Using a population genetics approach, we have discovered certain mouse lines that have unusual abilities to repair ear and knee (articular) cartilage after injury and are protected from developing post-traumatic osteoarthritis. While phenotypic differences have been identified, it remains unknown how differential response in healing takes place at cell and gene level. The recent work on chondrocytes and stem cells as well as transcriptome profiling of various knee joint tissues in the healer and non-healer mouse lines unraveled cell and transcript level cues as to why some individuals can repair their damaged or loss tissues and are protected from osteoarthritis while others cannot repair their injured tissues and are susceptible to osteoarthritis.
Osteoarthritis affects 3-4% of human population and is one of the leading causes of disability. At least 12% of osteoarthritis is due to trauma to knee and is the focus of my research. The studies on post-traumatic osteoarthritis have been undertaken with the overall goal to underpinning of molecular changes immediately after the injury but before the onset of clinical disease characterized by cartilage fibrillation and loss, synovitis and narrowing of joint space width. This approach of understanding the disease before the clinical manifestations is of paramount significance in translational medicine as one of the main reasons why no effective therapy is available for osteoarthritis is the lack of understanding of the early (molecular) changes in the knee which occur 10-15 years before clinical disease and diagnosis.
Currently osteoarthritis is diagnosed at the end stage where no therapy is possible and surgical joint arthroplasty is the only answer. Thus, if we can move the focus from end-stage disease to early molecular detection, we can better understand the disease process and can identify a window of opportunity for devising new therapeutic candidates as well as new therapeutic targets. To this end, using RNA sequencing we have probed the transcriptomic landscape of cartilage, meniscus and ligaments in patients with knee injury and have identified novel therapeutic targets. Moreover, to gain mechanistic insights into the tissue injury and osteoarthritis, we have developed surgical (invasive) and mechanical (non-invasive) mouse models of meniscus and ligament injuries. These animal models allowed us to study the course of post-traumatic osteoarthritis and to test various therapeutic regimens.
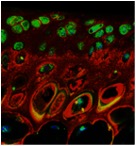
Nanoparticle-RNAi targeting periostin for the treatment of osteoarthritis
Matricellular and matrix proteins, in particular periostin (Postn), are emerging as promising pharmacological targets for osteoarthritis. Postn is a secretory matricellular protein that plays a critical role in tissue development, repair, and disease, but little is known about its function in synovial joints. While we have strong evidence that Postn plays a critical role in the early stages of osteoarthritis, understanding the therapeutic potential of modulating Postn across the spectrum of age and disease severity, as well as elucidating the mechanisms of Postn effects, is essential to design and implement mechanism-based clinical interventions.
Mechanistically, we reported that suppressing Postn dampens the inflammatory NF-kB/MMP-13 signaling axis in mice after destabilization of medial meniscus and in human chondrocytes in response to IL-1β, suggesting that Postn displays inflammatory properties. Yet the underlying mechanistic details of Postn-related OA effects remain obscure, and the interplay between intracellular inflammatory cascade and Postn was not fully elucidated by these earlier studies. To this end, we have established that (a) activation of NF-kB classical kinase (IKK2) and expression of its downstream inflammatory signature IkBz are essential for osteoarthritis development, and (b) IkBz is a potentially critical player in the inflammatory response and osteoarthritis cartilage degradation, suggesting that IkBz may be a superior therapeutic target due to its inflammatory gene signature. We anticipate that these studies will yield novel mechanistic insights regarding the role of Postn in osteoarthritis and demonstrate the strong translational potential of Postn as a therapeutic target for osteoarthritis.

Selected Publications
Current Membership in Professional Organizations
Although there are currently no open positions, aspiring PhD candidates or researchers interested in ongoing projects are encouraged to email their CVs and expressions of interest.