- Admissions
- Academics
- Research Office
- Student Life
- News & Events
- Outreach
- About
Dr Mayssa Hachem joined Khalifa University as Assistant Professor in department of chemistry leading the forensic chemistry track in January 2021. She graduated from National Institute of Applied Sciences ‘INSA’, Lyon, France with a PhD in Biochemistry and a distinction Award for higher education in Biochemistry. During her doctoral studies, she conducted research at the Cardiovascular, Metabolism, Diabetology and Nutrition ‘CarMeN’ research unit within the Engineering and Function of Lipids and Lipoproteins ‘InFoLiP’ team. She also served as a lecturer at the Biotechnology and Biochemistry Department at INSA. After her PhD, she undertook postdoctoral work at Sorbonne University, University of Technology of Compiegne ‘UTC’, France within the Enzymatic and Cellular Engineering research center ‘GEC’ Royallieu. She joined Amity University Dubai in 2017 as an Assistant Professor and Research Coordinator in the Forensic Science Department where she was extensively involved in the development of the Forensic Science undergraduate program. In 2020, she joined the Faculty of Health Sciences at the Higher College of Technology, UAE before moving to KU.
During her academic career, she has supervised a number of Bsc, Msc and PhD research projects and served on various academic and professional committees. Her main research interests concern lipid biochemistry, drug targeting and forensic science.
Faculty Start Up- FSU 8474000365 Title:Production of Labeled LysoPhospholipids with Docosahexaenoic Acid and study of their cerebral bioavailability Docosahexaenoic acid (DHA) is the main omega-3 polyunsaturated fatty acid in brain tissues and is required for normal brain development and function. Deficiencies in DHA have been associated with neurodegenerative diseases like Alzheimer and Parkinson diseases. Because DHA bioconversion from its precursor, alpha-linolenic acid (ALA or 18:3n-3), is limited in mammals, and does not compensate DHA deficiencies in brain, an exogenous supply of DHA is necessary for treating these diseases. Therefore, new therapeutic strategies to target the brain with DHA are needed taking into consideration the difficulties associated with delivering materials across the highly restrictive blood–brain barrier (BBB).
We previously showed that 1-lyso,2-docosahexaenoyl,glycerophosphocholine LysoPC-DHA is a preferred physiological carrier of DHA to the brain but not to other organs such as the liver, kidney, and heart. Also, several researches highlighted the cerebral accretion of LysoPC-DHA whereas the brain bioavailability of other forms of sn-2 DHA-Lysophospholipids LysoPLs including lyso-phosphatidylethanolamine LysoPE, lyso-phosphatidylserine LysoPS are not well explored.
The aim of the present project is to develop and study the cerebral uptake of different LysoPLs-DHA including LysoPS-DHA, LysoPE-DHA and compare their bioavailability to LysoPC-DHA and non-esterified DHA.
Firstly, we will optimize the chemical synthesis of sn-2 LysoPC-[14C]DHA, LysoPS-[14C]DHA, LysoPE-[14C]DHA since these labeled phospholipids are not commercially available. The synthesis will consist of two phases: the first step will consider the chemical acylation with [14C]-DHA of sn-2 position of commercial 16:0-LysoPC, 16:0-LysoPE, 16:0-LysoPS whereas the second step involves the enzymatic hydrolysis of fatty acids at sn-1 position leading to the production of LysoPL-[14C]DHA. Through the chemical synthesis and products purification, several analytical methodology will be applied including thin layer chromatography, TLC-linear analyzer, column chromatography, high performance liquid chromatography HPLC as well as mass spectrometry MS. Then, we will study the passage of synthesized lipids through a human model of Blood-Brain-Barrier purchased by Blood-Brain-Barrier Research Unit at Artois University, Faculty Jean Perrin, France, based on the use of hematopoietic stem cells and consisting of endothelial cells derived from stem cells in co-culture with bovine pericytes. After incubation with different LysoPL-[14C]DHA different compartments of BBB human model will be collected and radioactivity of total lipids will be quantified. Total passage of molecules across BBB will be evaluated through the sum of radioactivity found in lower medium and pericytes. The distribution of radioactivity between different lipid classes in cells and medium will allow better understanding of the metabolic pathway of each LysoPL-DHA. The mechanism of passage through BBB will be investigated to identify potential endothelial protein transporters.
The same study could be performed using 13C labeling and the purity of the synthesized product could be checked by HPLC, and 13C Nuclear Magnetic Resonance (NMR). The 13C enrichment could be determined by Gas Chromatography Combustion-Isotope Ratio Mass Spectrometry (GC-C-IRMS).
In summary, the proposed project consists of:
Optimization of synthesis of labelled sn-2 LysoPL-DHA
Study of the passage of labelled sn-2 LysoPL-DHA across BBB model
Identify mechanisms for DHA passage through BBB
The production of DHA-rich LysoPLs will therefore be of great relevance to pharmaceutical applications and will lead to new therapeutic targets for neurodegenerative diseases.
RIG 8474000575
Title: Production of economic and sustainable uniformly 13C labeled docosahexaenoic acid from heterotrophic marine protists Aurantiochytrium mangrovei and Crypthecodinium cohnii for medical applications
Docosahexaenoic acid (DHA, 22:6n-3), main polyunsaturated fatty acid in human brain and eyes, is essential for vision and normal brain development. Low levels of DHA have been associated with ocular diseases, including macular degeneration and glaucoma, as well as neurodegenerative diseases. Since DHA bioconversion from its precursor alpha-linolenic acid (ALA, 18:3n-3) in human is low, to compensate its deficiencies in these patients, therapeutic approaches targeting eyes and brain with DHA are required. To study DHA’s accretion and metabolism, researchers use stable isotopes (2H,13C) or radioisotopes (3H,14C) considered expensive and not easily accessible thus restricting studies to critical durations. The purpose of this study is to develop an economic and sustainable DHA biosynthetic production of uniformly labeled 13C-DHA in different natural forms through culture of two heterotrophic protists, Aurantiochytrium mangrovei and Crypthecodinium cohnii.13C-lipid molecules will be produced from 100 mg to 1 g for therapeutic applications in the medical sector.
Bachelor Final Year Project (CHEM 497- CHEM 498) Title: Forensic chemical investigation of acetylsalicylic acid-Aspirin According to Paracelsus (1495-1541) Swiss physician and chemist, “All substances are poisons. There is none which is not. The right dose differentiates a poison and a remedy.”
In post-mortem investigations, suspected drug overdoses are clear circumstances where toxicology is essential to identify if an excessive consumption of the drug occurred and, if so, whether this lead to death. On the other hand, toxicology testing can exclude the likelihood of a drug overdose if concentrations are not able of causing death, taking into consideration all other aspects. Several drugs are included in routine post-mortem toxicology such as analgesics (acetylsalicylic acid, acetaminophen), antidepressants, cannabis, cocaine, narcotic analgesics, etc. Forensic toxicologists employ numerous analytical techniques to determine the drugs or poisons significant to a death case investigation.
The objective of the present study is to develop several approaches to the quantitative determination of acetylsalicylic acid commonly known as Aspirin. Usually, Aspirin is used as analgesic agents for the treatment of mild to moderate pain and an antipyretic and anti-inflammatory agent for the treatment of soft tissue and joint inflammation. Fatal cases of oral aspirin overdose were identified in suicide or homicide cases in children and adolescents.
In the proposed project, Aspirin will be identified and quantified with the use of numerous analytical techniques including Thin-Layer Chromatography (TLC), High Performance Liquid Chromatography (HPLC), Liquid Chromatography-Mass Spectrometry (LC-MS), Nuclear Magnetic Resonance (NMR), Inductive Coupled Plasma Mass Spectrometry (ICP-MS) method for analysis of some inorganic elements or impurities such as aluminium, etc. Upon availability in chemistry laboratories, we will look for the isotopic profile or “signature” of Aspirin using Isotope Ratio Mass Spectrometry (IRMS) to provide unique insights into the origin of drugs and growing conditions which could helpful in forensic investigations.
For all mentioned experiments, Aspirin produced in several countries will be tested to confirm whether they have the same profile or not.
Altogether, these optimized approaches can be applied for the analysis of Aspirin in biological tissues (for example: hair) and fluids (blood, urine) in forensic post-mortem investigation. Since biological samples containing drugs/toxins cannot be injected directly into the analysing system without sample preparation, sample preparation prior to chromatographic/spectroscopic separation should be implemented to dissolve or dilute the analyte in an appropriate solvent, eliminating the interfering substances and pre-concentrating the analyte. In our experiments, we may use simulated blood trying to mimic the real biological fluids.
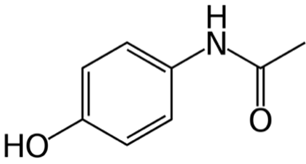
Bachelor Final Year Project (CHEM 497- CHEM 498) Title: DEVELOPMENT OF ANALYTICAL TECHNIQUES FOR DRUG PROFILING IN CRIMINAL FORENSIC INVESTIGATIONS
The aim of my project was to develop several approaches for illicit drug profiling. Due to challenges concerning the availability of illicit drugs, drug profiling was investigated with its physical and chemical aspects and applied on a common drug ‘Paracetamol’.
Several experiments were conducted for drug profiling of ‘Paracetamol 500 mg’ produced in five different countries: UAE, Saudi Arabia, Oman, Italy and Ireland. For physical profiling, several dimensions’ measurements and microscopic techniques including Stereomicroscopy and Scanning Electron Microscopy (SEM) were applied. For chemical profiling, Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and Liquid Chromatography-Mass Spectroscopy (LC-MS) were both utilized.
Significant differences were found in physical profiling of tablets produced in different countries. Tablets with cylinder shape (Saudi Arabia, Oman, Italy) were grouped together, and thickness as well as diameter measurements were found to be significantly different. For tablets with elliptic cylinder shape (UAE, Ireland), only diameter measurements were found to significantly vary.
For chemical profiling, ICP-MS was used to analyze 28 elements for 3 tablets from each origin. Many significant differences were determined between elemental compositions for tablets of different origin. For instance, Ca, Sr and Mn were found to distinguish tablets originating from Ireland. Furthermore, Bi, Ba and Ga were significantly different between tablets manufactured in Saudi Arabia and in Italy. Finally, through LC-MS, we observed differences between Area Under Curve. Altogether, our results provide full understanding of methodologies followed for drug profiling. Although a common drug was analyzed, same procedures could be applied for forensic analysis of illicit drugs.
https://orcid.org/0000-0003-1986-8304