- Admissions
- Academics
- Research Office
- Student Life
- News & Events
- Outreach
- About
Marwa joined the Department of Chemistry at Khalifa University as an assistant professor in 2022. During her Ph.D. work in Chemistry (Nanotechnology) at the University of Waterloo in 2015, Marwa’s research excellence was recognized by the Waterloo Institute of Nanotechnology (WIN) Fellowship and Queen Elizabeth II Graduate Scholarship in Science & Technology. Further, she was also recognized for her excellence in teaching several Chemistry and Nanoscience courses by the University Teaching Excellence Award at the University of Waterloo.
After her PhD, she worked in materials science as a postdoctoral fellow and research associate at the Chemistry, Chemical Engineering, and Electrical and Computer Engineering Departments at the Universities of Waterloo and Calgary in Canada. Her expertise is at the junction of physical chemistry and advanced materials characterization & development for a range of applications. She was awarded the Natural Sciences and Engineering Research Council of Canada (NSERC) and Global Research Initiative in Sustainable Low Carbon Unconventional Resources and Canada First Research Excellence Fund (GRI-CFREF) postdoctoral fellowships.
In 2020, She was appointed to a lectureship in Nanoscience and Nanotechnology at the Electrical and Computer Engineering Department at the University of Waterloo.
Silver nanowire (AgNW) networks have been widely touted as a promising material for transparent electrodes to replace indium tin oxide (ITO). Here we integrate polyurethane (PU) passivated AgNW electrodes into WO 3 /NiO electrochromic smart windows, placing particular focus on their NIR transparency. Experiments and modeling show that devices with NW electrodes permit more NIR radiation to transmit across the window compared to devices based on ITO. This is then shown to lead to a higher solar heat gain coefficient and lower U-factor, which is highly desirable in colder climates.
Electrochromic Smart Windows with High Near-Infrared Transparency Based on Passivated Silver Nanowire Electrodes
J Atkinson, M Abd-Ellah, IA Goldthorpe - 2The 23rd IEEE International Conference on Nanotechnology (IEEE-NANO 2023)
Two new π-conjugated polymers with donor backbone and π-conjugated indolin-2-one side chains, PBDTTI and PBDTTIF, are designed and synthesized as wide bandgap donors for non-fullerene acceptor-based organic solar cells (OSCs). The monomers containing electron accepting indolin-2-one side chains, (Z)-3-((2,5-dibromothiophen-3-yl) methylene)-1-methylindolin-2-one (M1) and (Z)-3-((2,5-dibromothiophen-3-yl) methylene)-5-fluoro-1-methylindolin-2-one (M2), can be easily synthesized via Knoevenagel condensation between 2,5-dibromothiophene-3-carbaldehyde and 2-oxindole or 5-fluoro-2-oxindole, respectively. Stille coupling polymerization of the electron-donating benzodithiophene (BDT)-containing monomer 1,1′-[4,8-bis [5-(2-ethylhexyl)-2-thienyl]benzo [1,2-b:4,5-b′]dithiophene-2,6-diyl]bis [1,1,1-trimethylstannane] and M1 or M2 produced PBDTTI or PBDTTIF, respectively. The strong electron accepting π-conjugated 1-methylindolin-2-one and 5-fluoro-1-methylindolin-2-one side chains can achieve low-lying HOMO energy levels of −5.59 eV for PBDTTI and −5.60 eV for PBDTTIF, which is beneficial for realizing high open circuit voltage (VOC) of the resulting OSCs. On the other hand, since the electron acceptor units are on the side chains, the polymer backbone containing only electron donor units could maintain wide bandgaps of 1.91 eV and 1.89 eV for PBDTTI and PBDTTIF, respectively. When PBDTTI and PBDTTIF were used as donors and a small bandgap non-full acceptor ITIC as an acceptor, the resulting OSCs devices achieved VOC of 0.97 and 1.00 V, short circuit current densities (JSC) of 15.60 and 13.70 mAcm-2, and fill factors (FF) of 0.60 and 0.59, resulting in power conversion efficiencies of 8.00 and 7.70%, respectively.
Novel Wide Bandgap Benzodithiophene-based Polymer Donors with Electron-withdrawing Indolin-2-one Side Chains for Efficient Organic Solar Cells with a Large Open Circuit Voltage
W Li, M Abd-Ellah, , Y Li, Dyes and Pigments 197, 109876, 2023
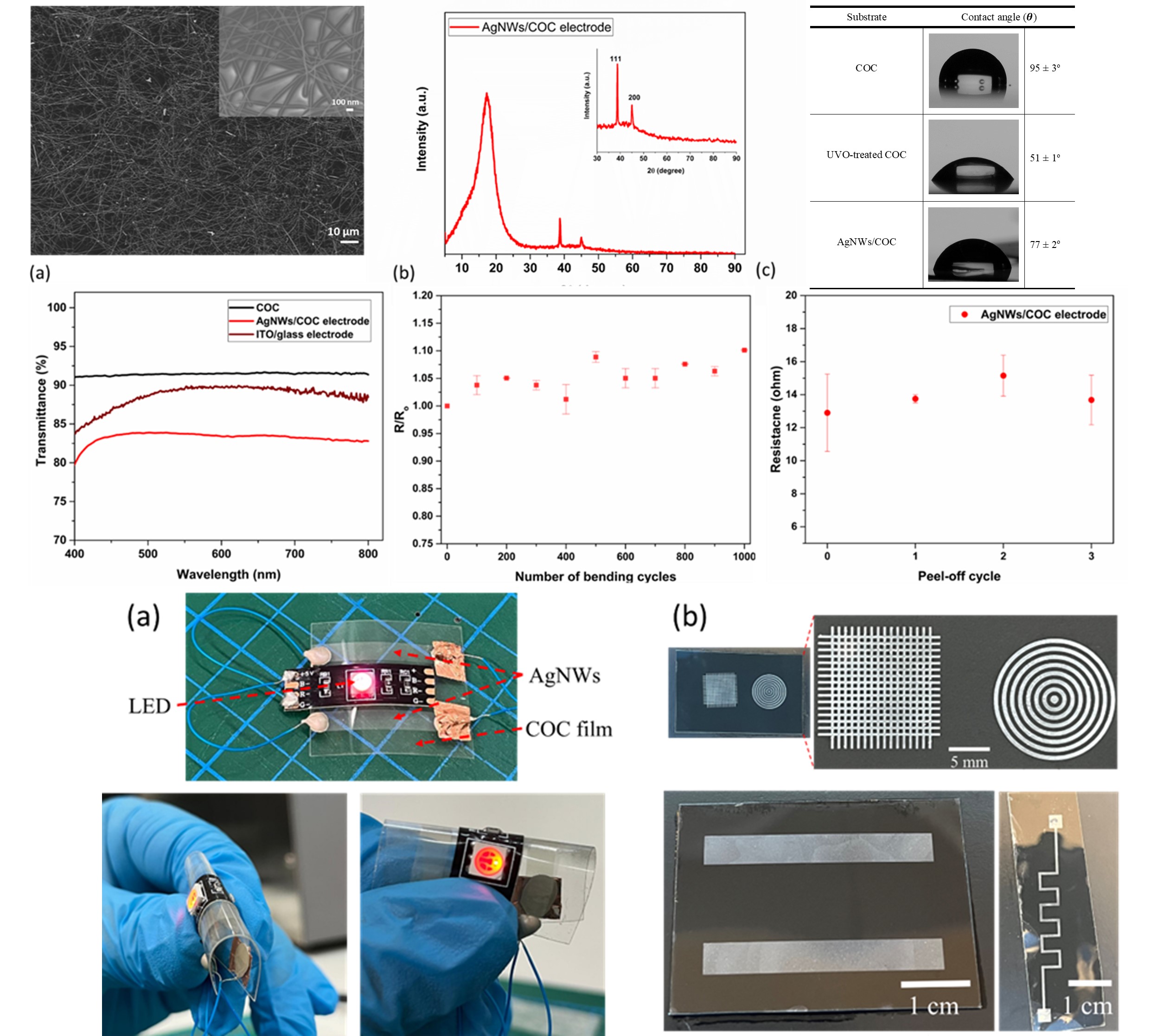
The development of flexible electronic devices has been a primary focus in various fields, and silver
nanowire (Ag NW) networks show significant promise due to their unique electrical and mechanical
properties. However, achieving well-defined and stable nanowire coatings on polymer substrates
remains challenging. This work presents a novel and simple approach for directly coating Ag NWs on
cyclic olefin copolymer (COC) substrates utilizing ultraviolet/ozone (UVO) treatment, a method not
previously demonstrated for this specific material system up to our knowledge. The compatibility of
this approach with COC eliminates the need for complex pre- and post-treatment processes, making
it a more straightforward and environmentally friendly way to improve adhesion between Ag NWs
and COC. The Ag NWs/COC electrodes exhibited excellent optoelectrical performance, with a high
optical transmittance of 84% and a low sheet resistance of 13 Ω/sq—metrics that compare favorably
to industry standards for transparent conductive films. Additionally, the Ag NWs/COC electrodes
displayed excellent mechanical stability, showing no changes in sheet resistance after both tape
adhesion and film bending tests. The novelty of the presented Ag NW-COC system, combined with
the simplicity and environmental benefits of the UVO coating approach, as well as the demonstrated
performance and stability of the resulting electrodes, make this work a significant advancement
towards realizing the commercial potential of flexible electronics for biocompatible and wearable
device applications.
Open vacancies for one PhD graduate student for research in experimental wet chemistry, materials science, and nanotechnology:
(1) Hybrid metallic and semiconducting nanomaterials for emerging technologies in solar cells, water desalination, and hydrogen generation; (2) Functional organic electronics in nanodevices and printed electronics.
Ph.D. student applicants must show interest and the right aptitude in working with high-tech, challenging projects and in learning about instrument systems and techniques at various research centers. For graduate admission information, please refer to: https://www.ku.ac.ae/graduate-admissions.
For Admission inquiries please email: pgadmission@ku.ac.ae
PDF applicants must have good hands-on and communication skills and must be able to lead in projects. For PDF hiring, please contact me directly via email: marwa.abdellah@ku.ac.ae