Research from Khalifa University is revolutionizing ammonia production with a novel catalyst
While hydrogen is a front-running potential solution to the world’s energy needs, production capabilities lag behind the predicted surge in demand. Ammonia (NH3) derived from hydrogen, however, could be a viable catalyst for hydrogen adoption. Its potential lies in its high hydrogen content, ease of liquefaction, and low transport costs.
A team of researchers from Khalifa University has designed and developed a novel catalyst to produce ammonia in a carbon-free process. Dr. Dinesh Shetty, Dr. Kayaramkodath Ranjeesh, Dr. Abdul Mohammed, Safa Gaber, Khaled Badawy, Dr. Mohamed Aslam, and Dr. Nirpendra Singh collaborated with researchers from Indian Institute of Technology Ropar; University of Strasbourg-CNRS, France; and University of Nova Gorica, Slovenia. Their catalyst showed exceptional performance, surpassing current benchmarks and meeting the targets set by the US Department of Energy for ammonia production. The research team published their results in Advanced Energy Materials.
The conventional method of producing ammonia, known as the Haber-Bosch process, is energy-intensive, requiring high temperatures and pressures, and relies heavily on fossil fuels, particularly for production of the hydrogen used in the process. This process is responsible for significant carbon dioxide emissions and consumes a considerable amount of energy. Alternative methods to the Haber-Bosch process include electrochemical nitrogen reduction, but such methods are not commercially adopted and face challenges such as low solubility of nitrogen in water and the competing hydrogen evolution reaction, which hinder efficiency and practical application.
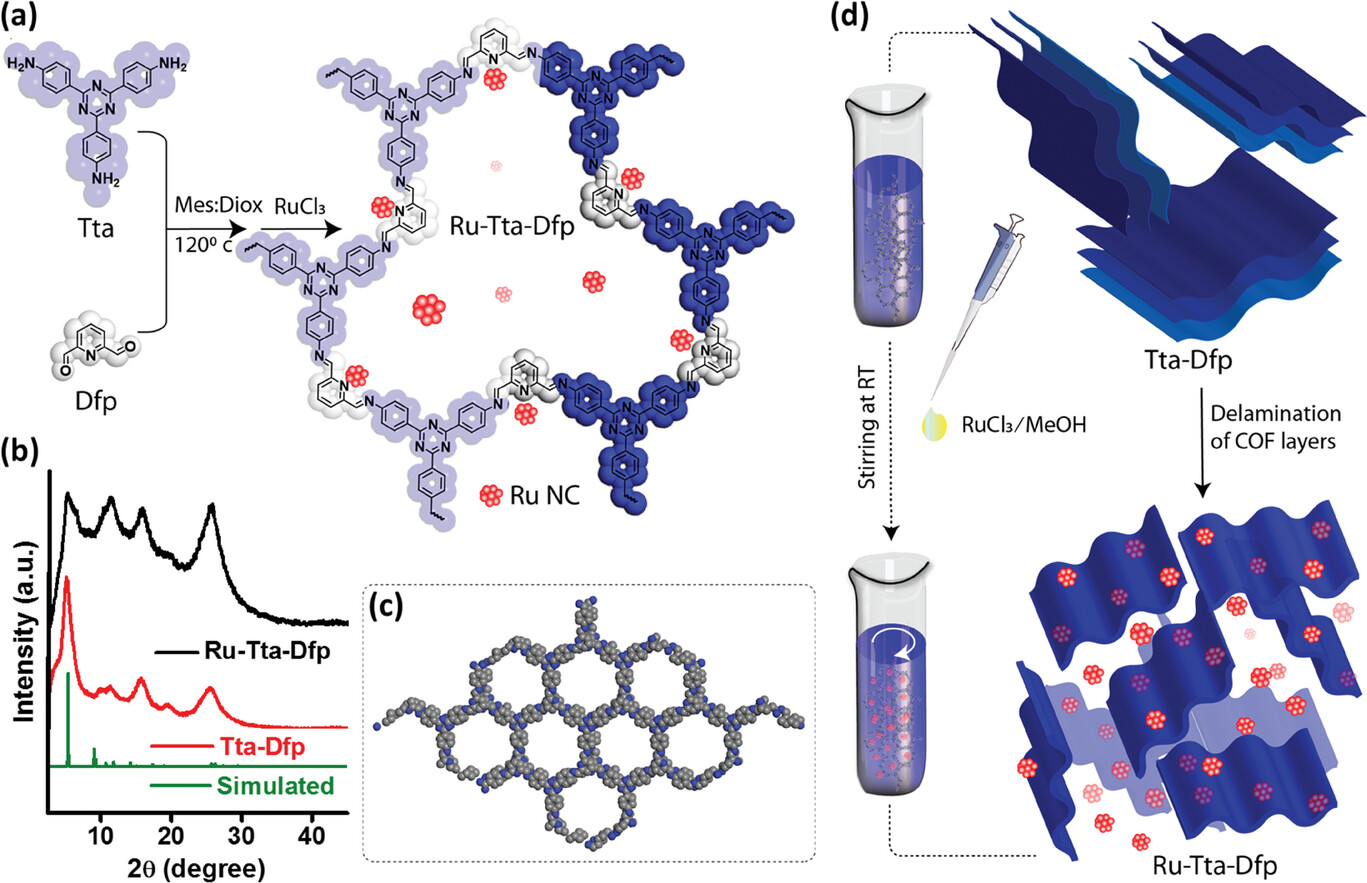
The research team overcame the limitations of the Haber-Bosch process by designing and developing a new catalyst that can more effectively facilitate the nitrogen-reduction reaction by minimizing the competing hydrogen evolution reaction. They used proton-filtering materials to increase the local concentration of nitrogen at the electrode surface, which enhances the selectivity of the nitrogen reduction reaction. The research team’s catalyst is a ruthenium-embedded, nitrogen-rich 2D covalent organic framework catalyst, which integrates proton filtration and catalytic activity within a single system. This innovative design not only simplifies electrode fabrication but also significantly improves catalytic efficiency.
“The ruthenium and strategic integration of nitrogen within the covalent organic framework are key to our catalyst’s success,” Dr. Shetty explains. “They enable effective interaction with both nitrogen and the protons within the reaction mixture. This suppresses the undesired hydrogen evolution reaction while promoting the nitrogen reduction reaction, resulting in the production of ammonia.”
The team’s novel catalyst offers the potential for the production of clean ammonia. A patent has been filed for their catalyst and the team is working on optimizing scalability.
“Importantly, the developed catalyst is easily scalable and opens the door for the translational opportunity given the breakthrough production efficiency,” Dr. Shetty says.
Jade Sterling
Science Writer
5 April 2024