Membrane-based organic solvent nanofiltration could save up to 90 percent of the energy required to reuse solvents in various industries. A team of researchers from Khalifa University’s Center for Membranes and Advanced Technology has developed a new nature-inspired membrane for this technique using holey graphene.
The broad spectrum of solvents with differing chemical and physical properties means there are myriad applications for their use. These solvents, for instance, are used in up to 90 percent of the processes used in the pharmaceutical industry. However, recovering these solvents for re-use typically requires energy-intensive processes.
Membrane-based organic solvent nanofiltration-separation techniques, however, could save up to 90 percent of this energy required, according to a team of researchers from Khalifa University’s Center for Membranes and Advanced Water Technology (CMAT).
Dr. Shadi Hasan, Associate Professor and Director of CMAT, Prof. Fawzi Banat, Professor of Chemical Engineering, Yazan Ibrahim, Research Engineer, Dr. Cyril Aubry, Dr. Parashuram Kallem, Postdoctoral Fellow, Mariam Ouda, PhD candidate, and Dr. Hanaa Hegab, Postdoctoral Fellow, developed an innovative next-generation two-dimensional membrane using holey graphene for solvent separation. They published their results in Chemical Engineering Journal.
“Compared to distillation and common evaporation techniques for organic solvent separation, membrane-based nanofiltration could save up to 90 percent of the required energy,” Dr. Hasan said. “The material required for such a membrane is critical: It should perfectly operate and maintain prolonged stability when used with harsh organic solvents during the separation process. In addition, this membrane should display high efficiency and precise selective capacity for small molecules at the nanoscale.”
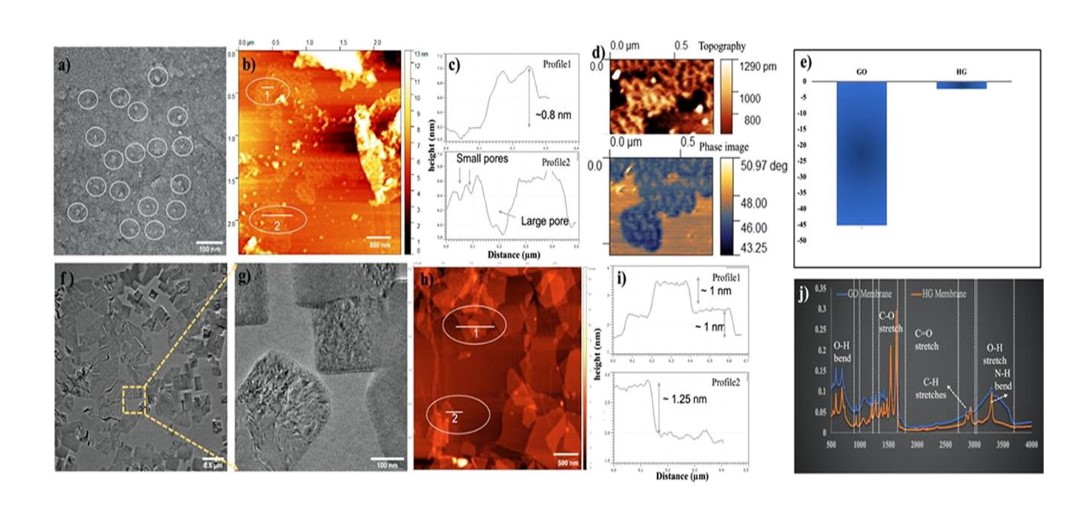
The research team used perforated, or holey, graphene with silicon dioxide nanoparticles to develop the membrane. In its purest form, graphene offers plenty of applications. However, in recent years, the nanoscale perforation of 2D materials has emerged as an effective strategy to enhance and widen the applications of the material beyond its pristine form.
Porous, or holey, graphene is a form of graphene with nanopores in its plane. The performance of the material is affected by the pore size, density, shape, and volume. Usually, uniform pore shape and size distribution is optimal as it leads to enhanced thermal, mechanical, and electrical properties. These pores are perfect for adsorption, where target molecules collect and attach to the surface of the pores. These features are all tunable too.
“Graphene membranes are already widely used in water treatment and organic-solvent nanofiltration processes because of their unique selective capacity, which can be governed by the tight interlayer 2D spacing among nanosheets,” Dr. Hasan said. “However, the pristine membrane without any chemical or structural modifications exhibits low permeation of organic solvents. Holey graphene can be produced with intentionally nanoscale holes to improve permeability while maintaining membrane selectivity for different applications.”
Using holey graphene also helps avoid other drawbacks seen with graphene and graphene oxide nanosheets: graphene oxide laminates suffer from swelling upon exposure to organic solvents, which reduces pore size and deteriorates the membrane selectivity.
“The limitation of the selectivity versus permeability trade-off in various membrane types, including graphene-based ones, remains a critical challenge in addressing practical applications,” Dr. Hasan said. “Studying the unique structures of microorganisms and their inimitable functions can lead to out-of-the-box solutions to real-world problems.”
There are biological membranes with high selectivity toward small ions and molecules, which can be attributed to fine-tuned multifunctional hierarchical nanochannels in their cellular membranes. These channels are known as aquaporins and they are a key component in biological membranes for boosting water permeability.
“The unique channel feature of aquaporin has inspired researchers to innovate a new generation of superior membranes,” Dr. Hasan said. “The protein molecules of aquaporin channels have alternative arrangements, including a hydrophilic region to guarantee high water permeability and a hydrophobic domain to enhance ion selectivity. Mimicking these unique features to develop a membrane with intentionally spaced interlayers and decorated with multifunctional groups will help tackle the crucial permeability/selectivity trade-off.”
The CMAT researchers designed a novel and facile strategy to fabricate aquaporin-like multifunctionalized membranes for efficient organic solvent nanofiltration. The interactions between the silicon dioxide groups and the holey graphene created a unique alternative 2D-spacing structure of nanoparticles, which allows high permeability and also high selectivity.
Their membrane design offers great potential for improving practical organic solvent nanofiltration in food, pharmaceutical, and other industrial separation applications.
Jade Sterling
Science Writer
15 November 2022