An innovative electrochemical process unlocks the potential of mining wastewater by recovering valuable metal ions and transforming them into valuable end products for a greener future.
A team of researchers from Khalifa University’s Center for Membranes & Advanced Water Technology (CMAT) and Catalysis and Separations (CeCaS) has developed a sustainable electrochemical process to recover metal ions in wastewater, turning them into valuable end products for energy conversion and storage applications.
Dr. Bharath Govindan, Research Scientist, Abdul Hai, Research Associate, Dr. Rambabu Krishnamoorthy, Postdoctoral Fellow, Dr. Mohammad Abu Haija, Associate Professor, and Prof. Fawzi Banat, Chair of the Chemical Engineering Department, published their work in a special issue of Resources, Conservation and Recycling.
“The UAE is a rapidly developing nation with a growing industrial sector,” Prof. Banat says. “As a result the country faces the challenge of treating wastewater from a variety of sources, including mining. Mining wastewater contains numerous suspended solids, dissolved metals, and other pollutants that can pose health and environmental risks. The UAE has enacted strict regulations and guidelines to ensure reliable and safe wastewater management. Our team has developed a strategy to recover metal ions, which are then converted into electrodes for commercial use in the energy sector.”
Using activated carbon derived from date seeds, a common waste product of the date fruit industry in the UAE, and a nitrogen-containing compound called polyaniline, the team developed an electrode that could be used to sustainably recover and reuse metal ions from mining industry wastewater.
“The recovery and reuse of metals in mining wastewater would not only ensure environmentally safe discharge of effluents, but also complement the metal demand for the mining industry,” Prof. Banat explains. “Acid-mining wastes containing highly acidic and toxic metal ions can be hazardous to aquatic life and the environment. While preventing these wastes from causing adverse environmental issues is crucial, the wastes can also provide an excellent source of metal ions for conversion into valuable end products.”
When rocks containing sulfide materials are exposed to air and water — often through mining — they undergo oxidation, producing sulfuric acid and dissolved iron. This acid can further dissolve other heavy metals such as copper, zinc, and lead present in the surrounding rock. These heavy metals can cause environmental damage if the wastewater is discharged without treatment. Prof. Banat points to the impact on waterways in particular: The high concentrations of iron and copper affect the smell, color, and oxygen balance of the aquatic ecosystem, killing fish and other aquatic organisms.
Recovering metal ions from wastewater is nothing new. Many countries have stringent regulations on the permissible limits of heavy metals in discharge streams, and there are techniques to treat liquid waste above acceptable levels. However, putting the recovered metal ions to reuse is a major challenge.
Mining wastewater often contains a complex mixture of metal ions, organic matter, and other contaminants, making it difficult to selectively recover and separate specific metal ions for reuse without also extracting unwanted impurities. Plus, the presence of multiple metal ions and other substances may interfere with the recovery process, leading to reduced efficiency and selectivity. Traditional methods are also limited by their robustness in varying wastewater conditions (frequent regeneration of replacement of the adsorbent material is time-consuming and costly) and their generation of secondary contaminants. Finally, once metal ions have been recovered using conventional techniques, they need to be further processed and purified before they can be reused. This can involve additional steps, which may be complex and resource-intensive.
Prof. Banat’s team thinks capacitive deionization (CDI) is the answer.
CDI is an electrochemical water-treatment technique. The process operates by electrosorption of ions on the surface of electrodes. When a voltage is applied between the two electrodes, the positive electrode attracts negatively charged ions (anions), while the negative electrode attracts positively charged ions (cations). As the ions approach the surface of the respective electrodes, they undergo electrosorption, where they accumulate at the electrode surface due to the electrostatic forces between the electrode and the ions. There they remain until the voltage is reversed or stopped.
By continuously cycling the applied voltage, the CDI process allows for the removal of ions from water without chemical additives or membranes, making it a promising technology for desalination, water softening, and water purification.
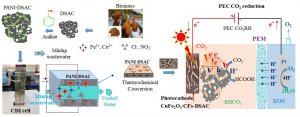
Fig. 1. Turning waste into wealth.
Separating highly valuable metal ions from mining wastewater can turn waste into wealth, says Prof. Banat, but it isn’t easy. The team needed to develop a novel electrode with an electrical double layer at the electrode-water interface to facilitate CDI.
The ideal electrode material for CDI applications has many features: It should have a large specific surface area for ion adsorption; exhibit high adsorption capacity, meaning it can attract and retain a significant amount of ions from the water; offer faster electrosorption kinetics for more efficient and quicker ion removal; have rapid conductivity to facilitate the movement of ions and ensure efficient charge transfer and overall performance; and be chemically stable to withstand the conditions and maintain its performance over time.
Carbon-based materials, including nitrogen-doped activated carbons, meet most of these requirements. Nitrogen doping in activated carbon enhances CDI performance through several mechanisms including the introduction of surface functionalities to enhance the overall ion adsorption capacity and increasing the charge density, which promotes the charge-transfer process during electrosorption. The presence of nitrogen also changes the structure of the carbon matrix, creating distortions that facilitate deeper ion access and charge accumulation.
Nitrogen sources such as ammonia, nitric acid, and polyaniline (PANI) have been used to synthesize nitrogen-doped carbon materials for CDI applications. PANI, in particular, contains amine and imine functionalities that significantly enhance the electrochemical adsorption of metal ions from wastewater.
The CMAT/CeCaS researchers used a material called polyaniline decorated date seed-derived activated carbon (PANI-DSAC), a composite of polyaniline and activated carbon made from date seeds. The seeds are a byproduct of the date fruit industry, which often generates a large amount of waste. Utilizing the seeds as raw material for activated carbon helps reduce this waste and promote sustainable resource management.
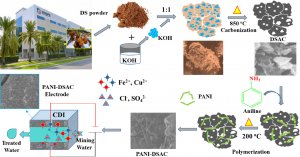
Fig. 2. Schematic diagram for preparation of PANI-DSAC composite.
This isn’t the first time the research group has used date seeds to produce activated carbon. In their previous work it was used to convert furfural into furoic acid and furfural alcohol via electrochemical hydrogenation. Date seeds are in large supply in the UAE and so the researchers collected the seeds at Khalifa University and reduced them to a fine powder before drying and heating to produce the activated carbon.
Besides the eco-friendly nature of using date seeds, the carbon produced from them has a highly porous structure, which provides a large surface area for adsorption. This makes it an ideal material for applications involving the removal of contaminants from wastewater, and date seed-derived activated carbon has been shown to exhibit good adsorption capacity for metal ions especially. Plus, they’re readily abundant and relatively cheap.
Considering the lower cost and favorable textural characteristics of intrinsically prepared activated carbon (specifically from sustainable green sources) compared with commercial activated carbon, exploring the use of intrinsic PANI-functionalized activated carbon for CDI-based recovery of metal ions is promising.
Date seed-derived activated carbon is a biodegradable and environmentally friendly material, making it a suitable choice for sustainable applications.
Furthering the sustainability of the process, once the metal ions have been removed from the wastewater, they can be put to good use in energy storage and conversion applications.
The CMAT/CeCaS team used the recovered metal ions to produce photocathodes for the photocatalytic conversion of carbon dioxide to formic acid.
“This work can help create a more sustainable future,” Prof. Banat says. “Environmental remediation strategies recover valuable metal ions from mining wastewater. These are used to make electrodes and electrocatalysts that can be used to convert carbon dioxide into fuels. CO2 capture and conversion technologies are being deployed to reduce emissions, while various renewable energy sources are being explored to reduce the nation’s dependence on fossil fuels. Formic acid is produced from CO2 using photoelectrochemical reduction strategies, and can be used in fuel cells to generate energy.”
The team has more work planned in this space, starting with research on different types of carbon electrodes for CDI and evaluating the potential for scaling up the process for industrial-scale metal ion removal.
Jade Sterling
Science Writer
22 May 2023