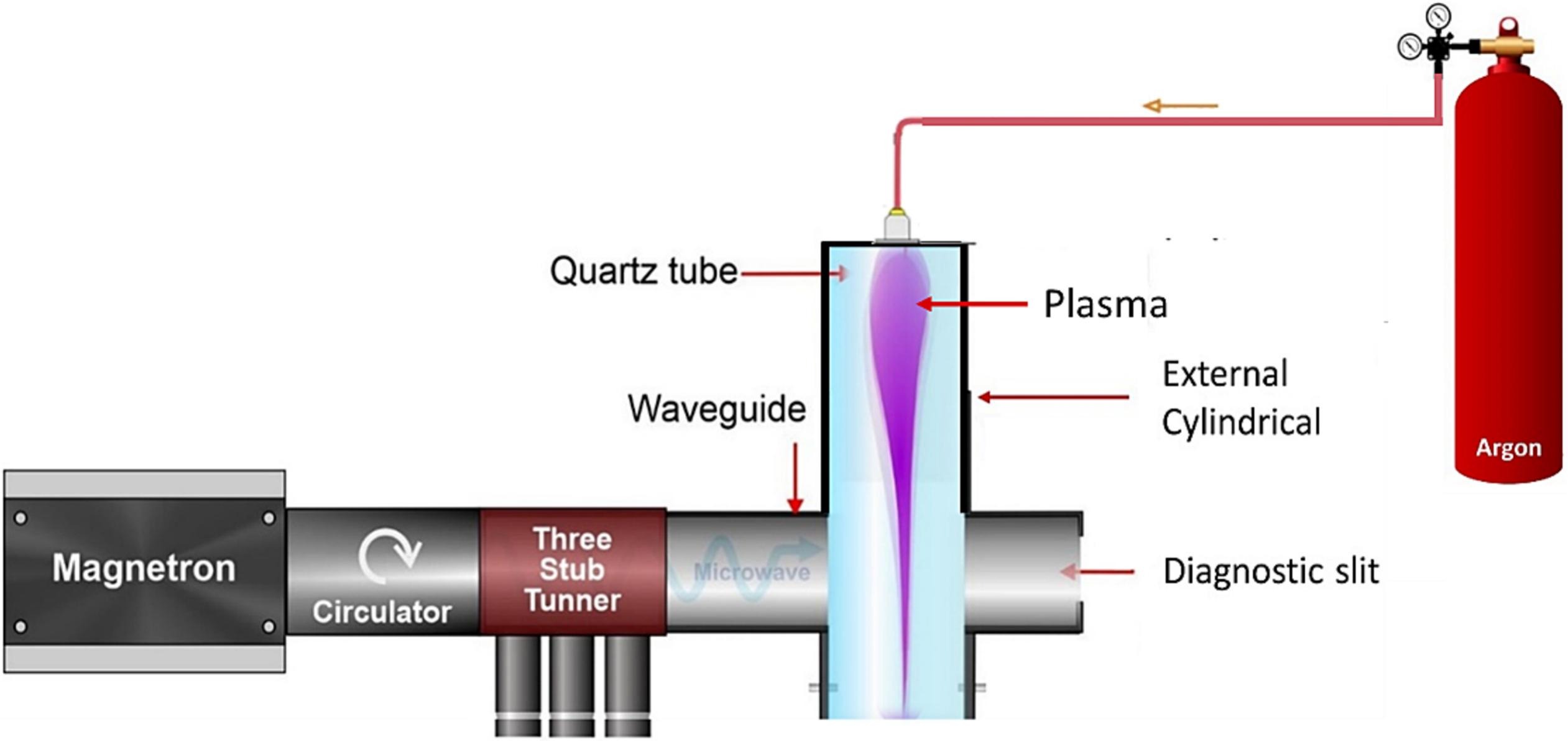
Plasma technology offers a novel approach to converting CO2 into major projects, but designing plasma reactors requires a comprehensive understanding of plasma parameters.
As carbon dioxide (CO2) emissions continue to rise around the world, innovative technologies for mitigation are in high demand. Carbon capture and utilization technologies aim to convert carbon dioxide into fuel and other valuable chemicals and a research team including Khalifa University’s Prof. Pau Loke Show is exploring the potential of microwave plasma technology for CO2 conversion.
The team simulated a microwave plasma reactor model to analyze various plasma parameters, providing critical insights into optimizing this technology for practical applications. They investigated key plasma parameters such as electron potential, density, and temperature at different pressures over time. The study revealed that while electron density eventually stabilized, the electron temperature and potential showed varied behaviors. This information is crucial for designing effective plasma reactors, especially for CO2 conversion.
Prof. Show collaborated with researchers from Universiti Tenanga Nasional, Malaysia; Nanyant Technological University, Singapore; and Central Queensland University, Australia. Their results were published in Applied Energy, a top 1% journal for engineering and environmental science.
“Carbon dioxide mitigation is of utmost importance,” Prof. Show says. “Numerous renewable energy technologies have been developed to do this and conversion using plasma is an emerging technology attracting increasing interest.”
Plasma is often called the “fourth state of matter” after solid, liquid, and gas. When gas is sufficiently heated, the molecules get more energetic and excitable, moving around more and more freely. At a high enough temperature, the atoms themselves will break apart, with electrons separating from their nuclei, leaving behind charged particles known as ions amid a swirl of electrons. This is plasma.
When other gases (such as carbon dioxide) interact with plasma, a chemical reaction occurs.
“This chemical reaction takes place in a plasma reactor,” Prof. Show explains. “Most plasma reactors comprise a pair of electrodes that create an electric discharge. This then energizes the electrons in the gas molecules, which causes them to travel incredibly quickly and bump into other gas molecules. As a result, they are broken down into atoms.”
Plasma conversion facilitates chemical reactions under milder conditions compared with traditional methods, and microwave plasma takes this a step further. Unlike other methods, which often require high temperatures, microwave plasma generates non-equilibrium (or cold) plasma and operates effectively at low or atmospheric pressure, making it safer and more energy-efficient. It uses microwaves to energize electrons in gas molecules, leading to the breakdown of CO2 with higher ionization power. This aspect is crucial for maintaining the structural integrity of certain compounds that might degrade under high heat, allowing for greater flexibility and control in the conversion process and selectivity in producing desired end products.
However, despite its promising aspects, microwave plasma technology faces challenges, particularly in adapting it for industrial-scale applications. The research team developed a model to simulate plasma parameters to help design plasma reactors for CO2 conversion. They highlight that exploring different gas inlets, pressures and the inclusion of catalysts in the plasma reactors could further optimize CO2 conversion and advocate for future research to focus on enhancing adaptability and efficiency. Expanding the application of microwave plasma to produce materials like graphene and industrial diamonds could also open new avenues for this technology.
Jade Sterling
Science Writer
26 January 2024






