International research team including Khalifa University paves way towards the design of new simple and efficient photocatalysts made from covalent organic frameworks (COFs) to reduce captured CO2 into useful products
As the world continues to pump carbon into the atmosphere, it is increasingly important to not only reduce emissions but also find ways to capture and use carbon dioxide. Carbon capture and storage technologies are noble approaches, but don’t tend to make much money. Instead, attention turns to economically viable and valuable approaches to turn carbon dioxide into something useful.
Dr. Dinesh Shetty, Assistant Professor of Chemistry, and Dr. Abdul Khayum Mohammed, Postdoctoral Researcher, collaborated with an international team to develop a new photocatalyst to efficiently and sustainably transform carbon dioxide into useful products. The research team comprised members from New York University Abu Dhabi, American University of Beirut, Instituto de Ciencia de Materiales de Madrid, Spain, University of Strasbourg, France, and University of Nova Gorica, Slovenia. The team’s results were published in ACS Applied Materials and Interfaces.
“Excessive anthropogenic emissions of carbon dioxide into the atmosphere have led to global warming,” Dr. Shetty explained. “At the same time, CO2 is a nontoxic, inexpensive, abundant, and renewable source of carbon. Converting it into high value-added products would be a viable and economic use of the carbon dioxide around us.”
Numerous processes already exist to transform CO2 emissions into various chemicals valuable for industry, and among these processes, photocatalytic reduction of CO2 has been noted as particularly promising. There’s little wonder why: this is photosynthesis. Green plants convert carbon dioxide and water into carbohydrates, performing this reaction under ambient conditions using just sunlight, which is an inexhaustible and environmentally-friendly energy source. Even better, photocatalytic CO2 reduction doesn’t create any secondary pollution.
“Carbon dioxide can be reduced into many forms, with carbon monoxide and formate the most common reduction products,” Dr. Shetty said. “Formate is preferred as it is the simplest oxygenated species produced, and an intermediate in the formation of methanol and other higher-order hydrocarbons, which can be used in plastics, paints, organic solvents, and fuel cells.”
Photocatalytic reduction of CO2 is not new—many semiconductor and molecular-based systems have been studied. However, their limited conversion efficiency, low binding affinity for CO2, unfavorable active-site architecture, and rapid charge recombination limit their overall performance. Covalent organic frameworks (COFs), such as that developed by the research team, have the potential to address many of these issues.
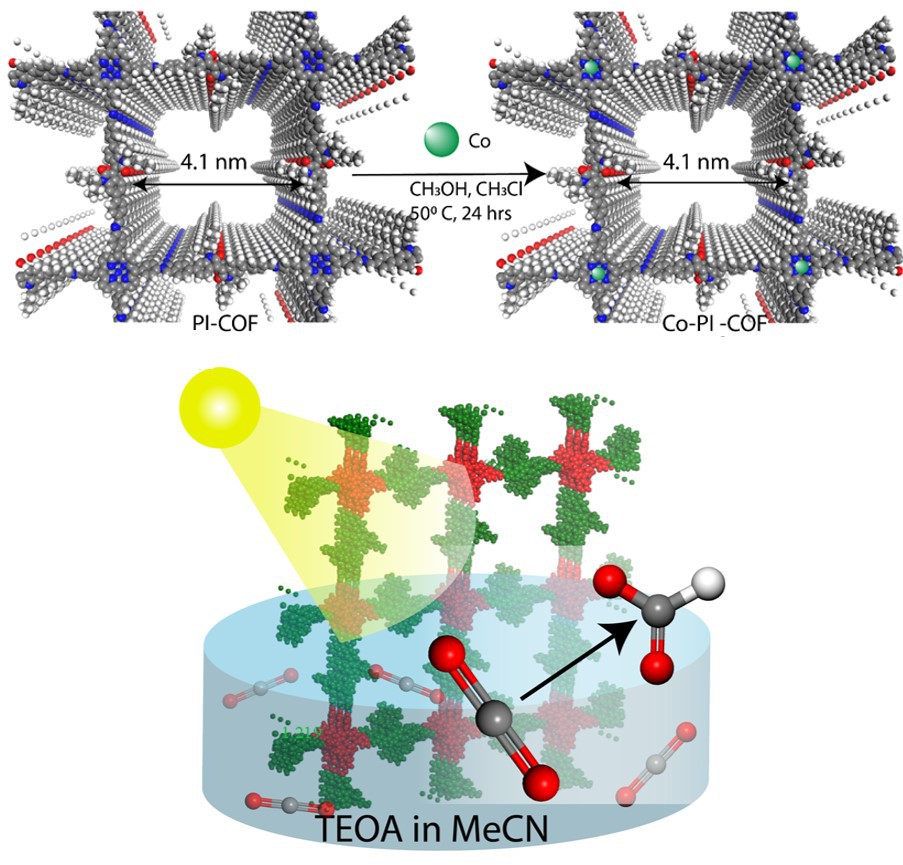
COFs are a class of materials that form two- or three-dimensional structures through reactions between their organic components, resulting in strong, covalent bonds that create porous, crystalline materials. They are uniquely tunable, with well-defined structures and good chemical stability and plenty of pores for adsorption applications.
Capturing the CO2 is the first step. A sorbent material is needed to selectively grab the carbon dioxide and allow it to collect in the pores in the material. And COFs for CO2 reduction already exist, but the majority produce carbon monoxide as their product, which is the less desirable of the two common products. Those that do produce formate often involve expensive noble metals or even enzymes.
The research team synthesized a novel COF using two different building units known as porphyrins and isoindigo to ensure the captured carbon dioxide reduces into formate, not carbon monoxide. Their PI-COF has a square layered structure and an improved affinity for carbon dioxide adsorption. Even without expensive rare materials or special catalysts, the research team’s PI-COF reduced carbon dioxide into formate with yields comparable to more complex systems.
“Our system performs similarly to others but requires much less power, making it a much more environmentally-friendly system,” Dr. Shetty said. “We expect this to pave the way towards more sustainable yet equally efficient photocatalytic systems for CO2 reduction.” Currently, Dr. Shetty’s team at KU is working on economically viable COF-based photoconducting materials for CO2 conversion.
Dr. Shetty is also a member of the Center for Catalysis and Separation (CeCaS), one of the research centers at KU.
Jade Sterling
Science Writer
4 February 2022






