Bioelectronics are anticipated to play a major role in the transition away from animal studies, offering a much needed technology to push forward the drug discovery paradigm.
An accidental discovery in 1928 marked a turning point in human history, when Dr. Alexander Fleming returned from a summer vacation to find his petri dishes of Staphylococcus aureus covered in mold.
Penicillin is famous as a serendipitous result, but for the first period of modern drug discovery, new drug discoveries primarily relied on luck and accidents. Nowadays, powerful techniques, including molecular modelling, automated high-throughput screening and recombinant DNA technology, allow us to develop potential drug candidates methodically and intentionally.
However, potential drug candidates must be tested under laboratory conditions “in vitro” before they can proceed to clinical trials in humans. In vitro is Latin for ‘within the glass’ and refers to work that is performed outside a living organism—such as in the petri dish on Fleming’s messy lab bench. In vitro methods are used to study bacterial, animal, or human cells in culture, providing a controlled environment for an experiment. The key challenge for cell-based in vitro models is to mimic, as accurately as possible, the state of the actual biological system being studied. Integrating electrical components offers an opportunity to noninvasively interface with these biological models for more accurate and quantifiable information.
Dr. Charalampos Pitsalidis, Assistant Professor of Physics at Khalifa University, reviews the advances in an emerging class of electronics made from organic electronic materials (conjugated polymers), for bridging the gap between the human body and the technology. The research team investigated the possibilities and challenges for conjugated polymers in clinical translation of in vitro systems involving biological models of varying complexity.
In this study, Dr. Pitsalidis and Dr. Anna-Maria Pappa, Assistant Professors of Physics and Biomedical Engineering at Khalifa University, respectively, collaborated with Prof. Owens’s team in Cambridge University and teams from University of Strathclyde and Universite de Lyon.
Their study was published in Chemical Reviews.
“In recent years, there has been a marked decline in the number of approved therapeutics, with attrition rates in drug discovery increasing at an alarming rate,” Dr. Pitsalidis said. “In addition, tighter safety regulations result in increasing development costs and decreasing profitability of new medicines, associated with the high costs of animal studies and their failure to predict adverse effects of promising drug candidates.”
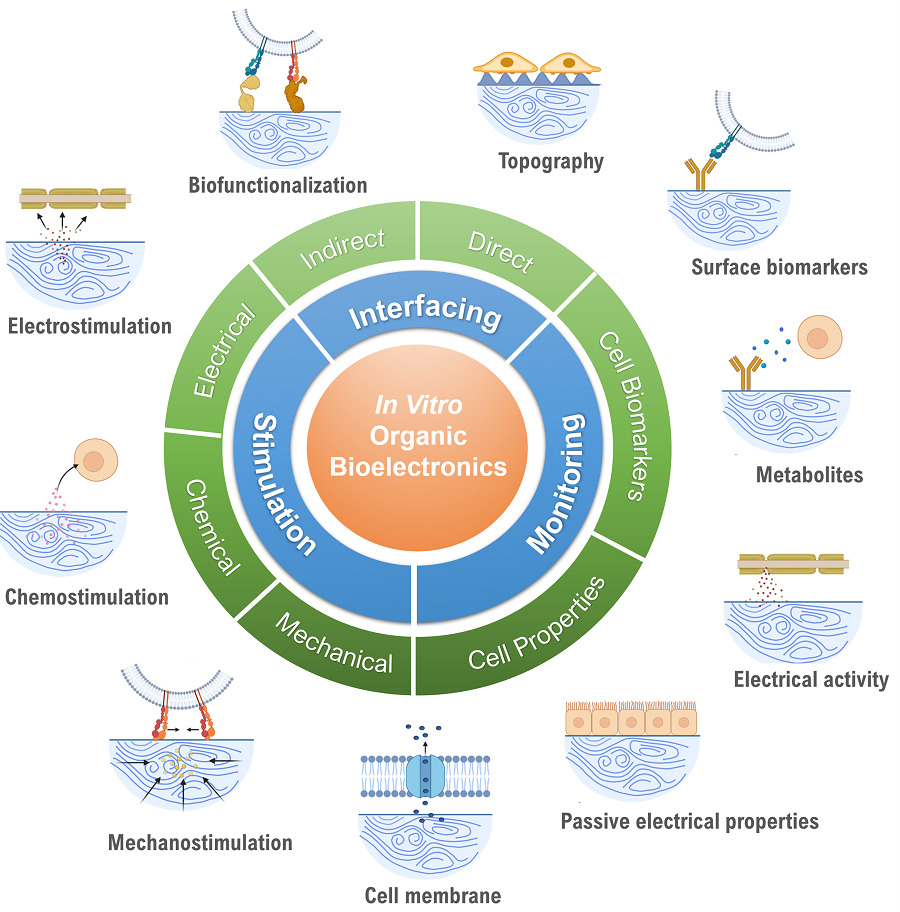
Fortunately, there are two key areas that can be investigated to improve success rates: we can focus on discovering new biomarkers and more specific drug targets, or we can improve our modelling technologies that better portray biology within full organisms, or in vivo biology, and allow us to test thousands of potential drugs quickly and accurately. Dr. Pitsalidis research team focuses on the development of new technologies for mimicking and monitoring biological systems as accurately as possible using organic bioelectronics technologies as reviewed in this work.
“Cell-based in vitro models have been increasingly adopted for applications ranging from tissue engineering to drug discovery and toxicology,” Dr. Pitsalidis said. “Besides being ethically advantageous, they are faster and more cost-effective, and can be easily standardized and validated. Advances in 3D cell cultures and the advent of microfluidics have heralded a new era of in vitro models, but there are some issues with the authenticity and validity of these systems. Plus, we currently lack a standardized and adaptable technology for meaningfully converting biological signals to a readable output.” In this regard, incorporating biosensors for in situ sensing of metabolites or critical biomarkers in the biological systems, will result in more accurate and holistic in vitro systems, critical for clinical translation said Dr. Pappa.
Key to developing these new technologies is a fundamental understanding of the interface between electronic materials and biology. Organic electronics are devices containing carbon and are anticipated to play a key role for biointerfacing—bridging the gap between the biotic and the abiotic.
“The advent of microfluidics and the considerable advances in reliability and complexity of in vitro models promise to eventually significantly reduce or replace animal studies, currently the gold standard in drug discovery and toxicology testing,” Dr. Pitsalidis said. “Organic electronic materials, notably conjugated polymers, have demonstrated technological maturity in fields such as solar cells and light emitting diodes, and are the obvious route forward for bioelectronics due to their biomimetic nature.”
Recent endeavors have seen organic electronic materials used in biologically relevant ion sensing, ion pumps and transducers of neural activity. They more seamlessly integrate with complex biological systems and offer more effective signal transduction of biological events.
Conjugated polymers are mixed conductors. The electronics surrounding us in our daily lives use electrons as the dominant charge carrier; biological systems use ions. Conjugated polymers can use both, which makes them a logical choice for direct coupling with biological systems.
“Typically, interfacing has been thought of as two-sided: stimulation on one side and monitoring on the other,” Dr. Pitsalidis explained. “We introduced a third component, where the chemical or physical characteristics of the active layer of the device can alter the biological system being studied.”
Interfacing can be a powerful means of controlling biological systems when used carefully. However, direct contact with biological tissue poses specific complications, and the set of requirements that a conjugated polymer has to meet is demanding in order to noninvasively exchange electrochemical signals. The polymer and the tissue must not damage each other. Aside from their electronic properties allowing them to be used in bioelectronics, they must remain stable for many cycles of operation, be flexible enough for a wide range of applications, and avoid injurious effects to biological systems.
“Most conjugated polymers are inherently biocompatible because they are mainly made of chemical elements that match the organic composition of cells and tissue, such as carbon and hydrogen,” Dr. Pitsalidis said. “However, biocompatibility cannot be universally defined because they can elicit different biological responses depending on the type of cells and the local tissue environment. Additionally, they are often modified or mixed with additives, which could be harmful to the in vitro system.”
The research team believes three-dimensional conjugated polymer-based scaffolds have the potential to be integrated with microfluidics to meet all the requirements of in vitro drug discovery.
“Now is the time to push forward accurate and reliable in vitro models that truly represent the in vivo situation,” Dr. Pitsalidis concluded. “We expect that the next few years will see conjugated polymers meeting all the scalability, accuracy and reliability requirements to replace animal models in drug discovery and disease research.”
Jade Sterling
Science Writer
19 January 2022