Despite the overwhelming demand for nanoscale technologies, little research exists on the effects of these materials on marine environments, with concern growing for their ecotoxicological impacts on aquatic species.
Researchers have previously looked at the impacts of individual nanoparticles on aquatic species. Now a team including Khalifa University’s Prof. David Sheehan has found a combination of the most commonly manufactured nanoparticles has a detrimental effect on a species of clam.
Prof. Sheehan, Dean of the College of Arts and Sciences, published the results in Science of the Total Environment, with authors Ilaria Marisa, Maria Gabriella Marin and Valerio Matozza, University of Padova; Davide Asnicar and Marco Parolini, University of Milan; Nicola Brianese, Institute for Energetics and Interphases, Italy; and Maria Fedorova and Ralf Hoffman, Universitat Leipzig.
“Organisms are exposed to complex mixtures of contaminants in their environments, not just individual substances,” Prof. Sheehan explained. “The challenge for ecotoxicological studies is to understand the potential combined effects induced by exposure to these mixtures. For aquatic species, there has been plenty of attention given to mixtures of pharmaceutical compounds, such as those in personal-care products, and the pollutants found in herbicides and pesticides, but little is known about the impact of manufactured nanomaterials in marine environments.”
Metal and metal-oxide nanoparticles are useful in a wide variety of commercial applications and consumer products, with manufacturers taking advantage of their unique electrical, optical and catalytic properties. Nanoparticles are extremely small fragments of matter with a diameter less than 100 nanometers, roughly one thousand times smaller than a single strand of human hair. Their unique properties stem from their small size, but these properties may also pose problems for marine coastal areas, which are a sink for chemical and physical contaminants, including nanoparticles.
“Laboratory toxicity studies on freshwater and marine species have shown the potential adverse effects of nanoparticles in the environment, but since information on their concentrations in marine areas is still lacking, we can only use predicted environmental concentrations as a reference,” Prof. Sheehan said. “Animals in these environments can easily come into contact with nanoparticles through their diet, and they can accumulate in the body, leading to oxidative stress, damage to DNA, protein and organelles, and even changes in gene expression.”
Organisms that have a sedentary lifestyle and feed on material suspended in the water may be particularly susceptible to nanoparticles in the environment. This includes bivalve organisms such as clams, oysters, and mussels, which filter large quantities of water to capture and ingest food particles. They play a critical role in cycling nutrients between the sediments and the water with a profound influence on water quality.
Because they are an important contributor to an aquatic environment, determining the impacts of nanoparticles on this group of animals is important for a full understanding of how manufactured nanoparticles might affect marine and coastal environments. Additionally, if nanoparticles are accumulating in bivalve tissues, they may be transferred to other animals that eat them, including humans.
“Although nanoparticles are present in aquatic environments in small quantities, so far, their mixture could cause different effects compared to each single nanoparticle,” Prof. Sheehan said. “A mixture of nanoparticle pollutants could interact in many different ways to induce biological responses at different levels of biological organization. There is also the potential ‘Trojan horse’ effect, which implies the facilitated uptake of toxic molecules adsorbed onto nanoparticles into the cells. Investigations on the combined toxic effects of multiple chemicals in organisms are much more challenging than those regarding single compounds, meaning information is lacking. Plus, the number of possible combinations of pollutants is extremely large and we don’t know which combinations would be the most ecotoxicologically relevant.”
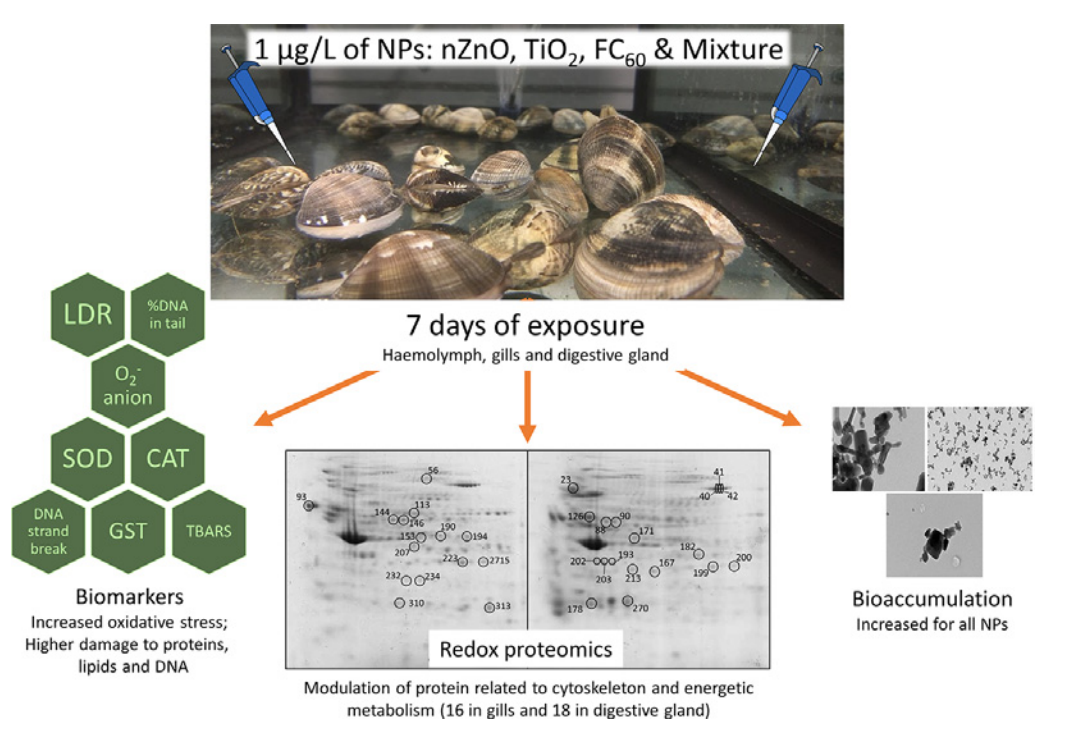
The research team investigated two metal oxide nanoparticles — zinc oxide and titanium dioxide — and a carbon nanoparticle (FC60) to assess their effects both independently and in mixture. These nanoparticles are the most produced and most commonly used in products worldwide, and while many studies have been conducted to understand their impacts individually, this was the first to study their effects as a mixture. The team collected specimens of the marine clam Ruditapes philippinarum to test the effects of the nanoparticle mixture over seven days.
They used a multi-biomarker approach to better understand the effects of the nanoparticle mixture, and in all tissues analyzed, oxidative stress was the main mechanism influenced by nanoparticle toxicity. The research team found that during the early days of the investigation, the clam gills and digestive gland were able to cope with the presence of nanoparticles, limiting any damage, but after seven days, the mixture caused significant damage to the lipids and DNA in the digestive gland.
“Our findings indicated that the nanoparticle mixture had detrimental effects on the haemolymph, gills and digestive gland of clams, with the digestive gland most affected,” Prof. Sheehan said. “We also found that while concentrations differed, the nanoparticles did accumulate in the clams. Overall, the complexity of assessing the effects of contaminant mixtures was clear, and many environmental drivers and physiological constraints in various species need consideration in future studies.”
Jade Sterling
Science Writer
17 June 2022






