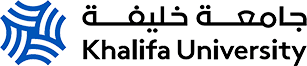How Khalifa University research into gas hydrates found in sediment over 13,000km away in the Gulf of Mexico can teach us how to recover the natural gas trapped in unconventional reservoirs here in the Middle East.
Gas hydrates in sediment have the potential to provide an important source of natural gas around the world. But extracting them is a problem, and finding ways to better extract them – and similar unconventional gas resources – requires better understanding of their characteristics.
That’s why a team of researchers, including Khalifa University’s Dr. Muhammad Arif, Assistant Professor of Petroleum Engineering, used X-ray microcomputer tomography (micro-CT) to investigate the characteristics of gas hydrates in sediment samples. They demonstrated the potential of micro-CT as a tool to better understand the distribution and formation of gas hydrates in natural settings, a technique that could have important implications for energy exploration and production.
The team published their results in Earth-Science Reviews, one of the top journals in the field of earth and planetary sciences.
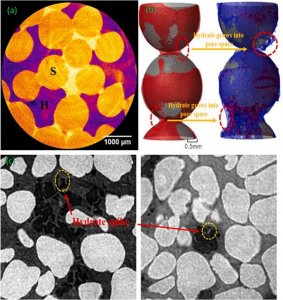
Fig. 1. Gas hydrate growth pattern in porous media using microcomputed tomography.
“Natural gas hydrates are one of the unconventional gas reservoirs which have shown enormous natural gas potential across the globe,” Dr. Arif explains.
But a precise characterization of their structure and pore habits is essential to successfully exploiting their reservoirs. “This requires the use of advanced imaging techniques,” He says.
Natural gas hydrates are efficient and promising energy resources because of their high energy density. They are crystalline solids, comprising gas molecules surrounded by a water molecule cage, with hydrogen bonds connecting the water molecules. The gas molecules are trapped in the cage and not bonded, held together by the van der Waals force. Usually formed by methane, other hydrocarbons such as carbon dioxide and hydrogen sulfide can also form a gas hydrate.
Understanding the physical properties of gas hydrates is crucial in designing processes to extract them. Multiple factors are at play: the hydrate type, its saturation degree, and the encaged guest molecule in the gas hydrate.
The water molecule cages are latticed structured, creating cavities to trap and encage guest molecules within the structure. In the case of methane hydrates, the methane molecules are the guest molecules enclosed within the water lattice. Each methane molecule occupies a single cage, and multiple cages are connected to form the overall structure of the gas hydrate. The encaged guest molecules in gas hydrates are significant because they contribute to the hydrate’s unique properties, affecting its stability, structure and physical properties, including density and thermal conductivity.
“Gas composition determines the structure, composition, and thermodynamic properties of gas hydrates,” Dr. Arif explains. “The gas composition also influences the kinetics and hydrate formation. Complex mixed hydrates have more than one component, in which cages of the same kind are occupied by two or more molecule types, with the restriction of, at most, one molecule per cage. Simple methane hydrates have only one guest species. Both complex mixed hydrates and simple methane hydrates have been recovered from natural sediments.”
There are two common hydrate types (structure I and structure II) and an uncommon type known as sH. Structure I is the simplest, comprising two cavities with 46 water molecules that usually trap methane, carbon dioxide, ethane and hydrogen sulfide. Structure II is slightly more complex, still with two cavities. However, structure II hydrates have 136 water molecules and the most common guest molecules are propane, nitrogen, isobutene, and tetrahydrofuran. In sH hydrates, there are three cavities and 34 water molecules. The major difference between structure I and II hydrates and sH hydrates, however, is that structure I and II hydrates require only one guest molecule while sH hydrates need two. These two are both a small molecule, such as methane, in small, medium, and large cavities, and a larger molecule in the large cavities.
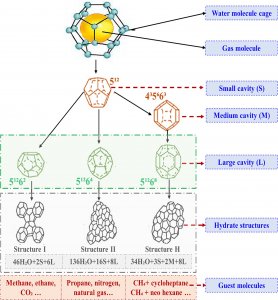
Fig. 2. Hydrate structure types: Structures I, II, and H with various molecular compositions
The research team used micro-CT to analyze the microstructure of the sediments and hydrates in sediment samples collected from the Gulf of Mexico. Micro-CT is used to image the samples at high resolution. It is a non-destructive imaging technique that works by taking multiple X-ray images of an object from different angles and using software to reconstruct a three-dimensional image of the object. In this way, the team was able to visualize the distribution of gas hydrates in the sediment samples and measure of the volume of hydrate in each sample.
“Our work presents the current state-of-the-art application of micro-CT to characterize natural gas hydrates,” Dr. Arif says. “I conceptualized this work and focused on the specific applications of X-ray microcomputed tomography to characterize the microstructure of the hydrates.”
The researchers found that the microstructure of the sediment in which the natural gas hydrates form has a significant influence on the formation and distribution of gas hydrates. Samples with a higher degree of heterogeneity in the sediment matrix tended to have more hydrates than samples with a more homogenous matrix. The researchers also found that the size and shape of the hydrate particles varied depending on the sediment matrix.
“The occurrence of gas hydrates depends on low-temperature and high-pressure conditions, where the presence of gas is sufficient to initiate and stabilize the hydrate,” Dr. Arif says.
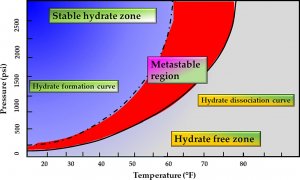
Fig. 3. Pressure-temperature curve of hydrate phases: hydrate formation and dissociation regions.
Natural gas hydrates can be found in many geological settings, such as within fractures and faults in rock formations, but they most commonly occur in sedimentary environments. Sediment refers to loose particles of various sizes that accumulate over time through erosion and deposition. They include a mixture of minerals, organic matter, shells and other materials, and are typically found in areas such as riverbeds, lake bottoms, and the seafloor. For gas hydrates, sediments provide the necessary conditions of low temperature and high pressure for their stability.
Gas hydrates require relatively low temperatures to form and remain stable. The insulating layers of sediment maintain the low temperatures and gas hydrates are more commonly found in sediments in deep ocean regions and Arctic permafrost areas. They also need high pressure and, in sediment, the weight of the overlying layers exerts pressure on the deeper layers. The decomposition of organic material in sediment produces the necessary methane as a byproduct, which is then trapped within a water lattice, forming the hydrate deposits.
The large amount of methane that can be stored in a relatively small volume of gas hydrate makes it an attractive source of natural gas. However, extracting and utilizing the methane from gas hydrates pose technical and economic challenges. A good place to start? Characterizing the gas hydrates and sediment to fully understand the scope of the challenge.
Fig. 4. Map presenting gas hydrate locations.
Dr. Arif says the use of advanced imaging and characterization tools, such as micro-CT, is gaining significant attention globally to solve scientific problems: more precisely, to elucidate the microstructures of a range of materials.
“For me, specifically, the materials of interest are the geo-materials, the subsurface rocks and rock-forming minerals,” Dr. Arif says. “The characterization of unconventional reservoirs is one of the areas my research group at Khalifa University is actively working on. In the future, we will be studying the applications of X-ray computer tomography to characterize the enhanced methane recovery by CO2 injection in shales.”
For Dr. Arif and researchers in the Middle East, sediment rocks and gas hydrates aren’t the main focus. While gas hydrates have been found in many parts of the world, including the Arctic, the Gulf of Mexico, and the South China Sea, there have been few studies focused on the presence of gas hydrates in the Middle East. The geology of the region is dominated by rocks that are not typically conducive to the formation of gas hydrates, and this part of the world is famous for its heat, not its low temperature waters.
There have been some reports of gas hydrates in the eastern Mediterranean Sea. Estimates of the volume of natural gas reach up to 1.7 trillion cubic meters, which has researchers in the Levant intrigued, but Dr. Arif is more interested in the tight sands and shales that also have “enormous hydrocarbon potential.”
“On a regional basis, the UAE has around 160 trillion cubic feet of recoverable unconventional gas resources, and it is estimated that an additional 1 billion cubic feet per day will be added to the UAE’s net natural gas portfolio via unconventional gas production by 2030,” Dr. Arif says. “However, characterization of such unconventional reservoirs is challenging and requires a multiscale imaging framework, which is where the applications of micro-CT come into play.”
By testing the techniques on natural gas hydrates in sediment rocks from miles away, Dr. Arif and his team can extrapolate the methodology to the geology of the Middle East. Gas hydrate reservoirs are difficult to tap into, much like the unconventional shale reservoirs here. Research into understanding the characteristics of an unconventional source of natural gas can only help drive hydrocarbon exploration and production further.
Jade Sterling
Science Writer
22 May 2023