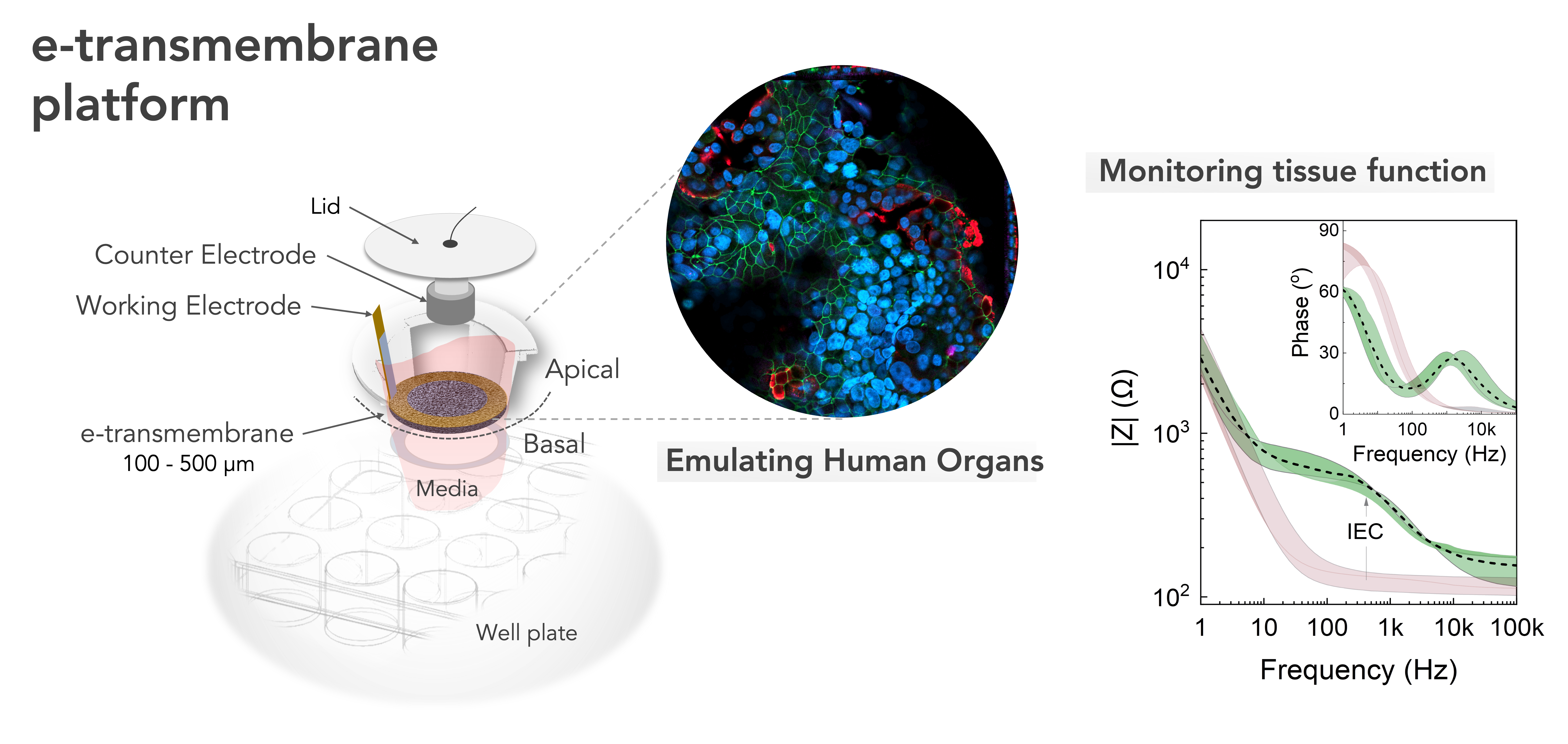
The new e-transmembrane platform supports and monitors complex 3D cell architectures and could be a universal resource to conduct next-generation drug-screening assays.
Re-creating the human body in a Petri dish isn’t exactly straightforward. In vitro methods are used to study bacterial, animal, or human cells in culture, isolating specific cells and structures to provide a controlled environment for an experiment. The key challenge for cell-based in vitro models is to mimic, as accurately as possible, the state of the actual biological system being studied.
In a new study, Dr. Charalampos Pitsalidis, Assistant Professor of Physics at Khalifa University, describes the development of a new bioelectronic platform to support and monitor complex 3D cell architectures. In collaboration with researchers from the University of Cambridge, Dr. Pitsalidis created an organ-on-a-chip device to better mimic human tissues and organs for a more in-depth understanding of how the cells interact with potential drug candidates.
The results were published in Science Advances.
“Current understanding of the growth, function, and homeostasis of cell and tissues mainly arises from two-dimensional (2D) cell-based assays using cell monolayers cultured on flat, rigid surfaces,” Dr. Pitsalidis explained. “While such assays have been applied for both fundamental research and toxicology screening, they cannot re-create the complex 3D microenvironment found in vivo.”
This re-creation is made possible using micro-engineered scaffolds to function as membranes, upon which the cells can build their tissue structures. These membranes also act as separators to compartmentalize and spatially organize multicellular cultures.
Integrating electrical components allows for the real-time recording of cell growth and function, offering an opportunity to noninvasively interface with these biological models for more accurate and quantifiable information.
The research team named their scaffolding platform the e-transmembrane. It is capable of supporting and monitoring complex 3D cell architectures and has the potential to become a universal tool for biologists for the next generation of high-throughput drug-screening assays.
“Using the e-transmembrane platform, we can generate high-complexity cellular constructs and monitor cell growth and tissue functionality,” Dr. Pitsalidis said. “It combines tissue engineering approaches with electronic components; the first promotes the development of cells in a 3D space, while the second allows for electrical monitoring of tissue functional properties. The e-transmembrane can be directly applied in the development of biologically relevant 3D cell models of various organs or disease states.”
Bioelectronics are anticipated to play a major role in the transition away from animal studies, offering a much needed technology to push forward the drug-discovery paradigm. The e-transmembrane device is compatible with well plate models, which represent the current standard for culturing cells in the lab, meaning it can be quickly and easily integrated into existing laboratory facilities and set-ups.
The scaffolding material used is a further advantage. Most scaffolds used for 3D cell cultures are based on natural polymers, including collagen, or synthetic polymers. Although these scaffolds support the 3D architecture, they present imaging challenges, making it difficult to evaluate the interactions with the cells. Using electroactive scaffolds based on mixed ionic-electronic conductor materials aids the development of bioelectronic devices that can more seamlessly integrate with complex biological systems and offers more effective signal transduction of biological events.
Additionally, while conventional rigid plastic membranes are limited to variations in the pore size and thickness, the e-transmembrane can be engineered for purpose by tailoring the biochemistry, porosity, conductivity, and stiffness.
The team tested the e-transmembrane platform by developing a 3D human intestine model and using the electrical components to detect breaches in the intestinal barrier. In certain conditions, the cellular network is compromised, which leads to breaks in the cell-barrier integrity. This can be identified by a decrease in the trans-tissue resistance as monitored by electrochemical impedance spectroscopy measurements.
Dr. Pitsalidis said. “In contrast to other in vitro biochips, e-transmembrane offers a simple approach to generate and simultaneously measure 3D cell cultures as a more accurate mimic of human physiology.”
The team plans to further their work by concentrating on expanding the repertoire of tissues modeled on the e-transmembrane to lung, blood-brain barrier, esophagus and vaginal epithelia for a host of drug-discovery and disease-modeling applications.
Jade Sterling
Science Writer
15 November 2022