Khalifa University Research Team Investigates the Future of Stem Cell Therapies
Read Arabic story here.
Researchers hope stem cells will one day be effective in treating many medical conditions and diseases for which few treatments exist given that stem cells offer the potential to repair, restore, replace and regenerate cells.
Despite the successes seen so far, there are several major challenges that must be addressed before stem cells can be used as cell therapies to treat a wider range of diseases. One of these challenges, investigated by a team from Khalifa University, is the pathway by which stem cells self-renew or differentiate.
In a review paper recently published in Stem Cells, Dr. Abdulrahim Sajini, Assistant Professor of Biomedical Engineering, along with Dr. James McElhinney, Post-Doctoral Fellow, and Dr. Ayesha Hasan, Assistant Professor of Biomedical Engineering, cover exciting insights into how small modifications to gene expression controls can influence this pathway.
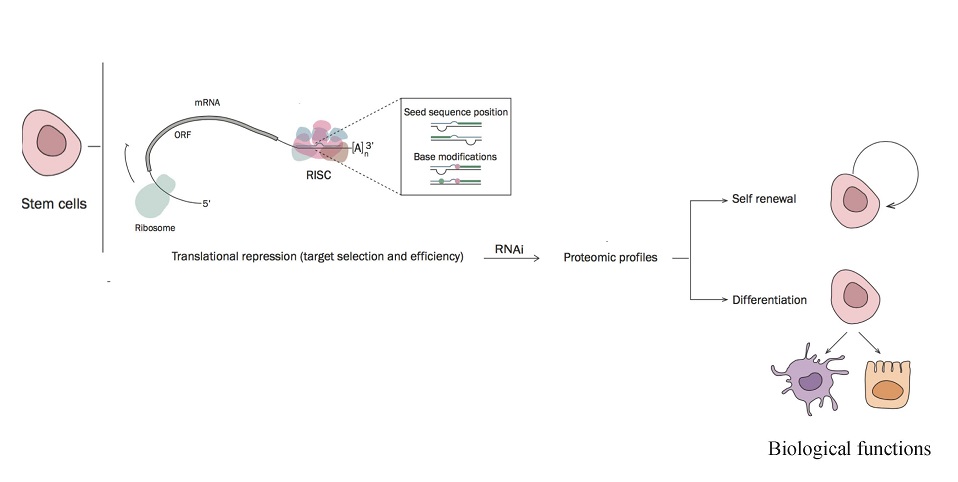
Stem cells are the foundation for every organ and tissue in the body. All stem cells can self-renew, dividing indefinitely to produce more of the same stem cell, or differentiate, developing into specialized cells.
“The unique properties of stem cells make them well suited for regenerative medicine,” explained Dr. Sajini. “Stem cell therapies have shown considerable promise in the treatment of a diverse variety of medical issues, including regrowth of cartilage for osteoarthritis, pancreatic beta cell regeneration for diabetes and neural stem cell transplant for spinal cord injury.”
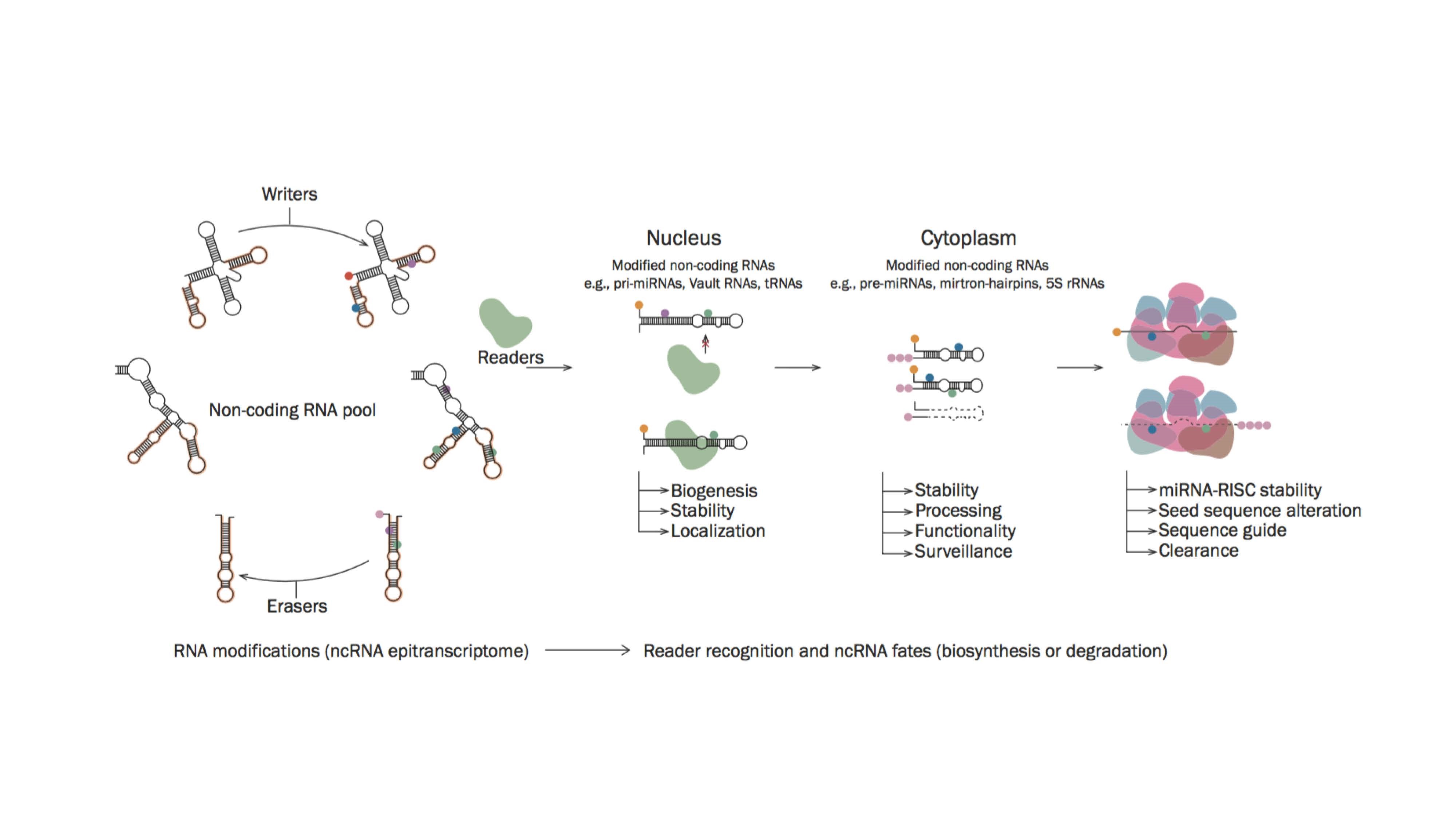
Before stem cells can be used more extensively in regenerative medicine, they need more study. Researchers need to first learn more about how stem cells behave and how they generate different types of human cells. The KU team reviewed the different types of stem cells and the unique epitranscriptome roles associated with them.
RNA is responsible for coding, decoding, regulating and expressing genes held within our DNA. Recently RNA was found to interact with various modifiers within our cells that can affect whether a gene is expressed or not. This is known as the ‘epitranscriptome’, which can be thought of as an extra layer of instructions for the RNA similar to epigenetics in DNA.
There are different types of stem cells, found during embryonic or adult development, with slightly different properties. Embryonic stem cells are the most pluripotent, meaning they can develop into any cell making up the body, which makes sense as all humans begin as embryos. However, this pluripotency requires multi-layer regulation check points, with the epitranscriptome playing a crucial role in maintaining the plasticity required for embryonic stem cells to self-renew or differentiate. In contrast, germline stem cells are unipotent cells that only give rise to haploid gamete cells.
“Stem cells are unique cells that have an inherent ability to self-renew or differentiate,” explained Dr. McElhinney. “Both fate decisions are strongly regulated at the molecular level via intricate signalling pathways. These pathways were thought to be governed by the action of transcription factors but now, small non-coding RNAs (ncRNAs) and their post-transcriptional modifications have emerged as additional regulatory layers with essential roles in this process.”
“Research into the epitranscriptome signatures in stem cells has revealed the emerging importance of an expanding set of ncRNAs in regulating cell biology. Moreover, these RNAs have been found to be extensively decorated with chemical modifications that comprise a complex regulatory layer on their functionality. As a fundamental governor of cell behavior, elucidating the epitranscriptome would represent a significant step forward to addressing challenges faced by stem cell therapies.”
There are many challenges associated with stem cell therapies. For example, the embryonic stem cells available today are likely to be rejected by the body; adult stem cells on the other hand are difficult to grow in the lab and can only be found in small quantities in their niche.
Currently, the only established therapy using stem cells is hematopoietic stem cell transplants to treat people with conditions such as leukemia and lymphoma—a bone marrow transplantation in most cases as hematopoietic stem cells are those responsible for making blood cells.
One important challenge is deciphering the imbalances in homeostasis that result in the development of cancer stem cells. Some stem cells form tumors after transplantation as pluripotency is linked to tumor formation. Recently, the epitranscriptome has emerged as a major regulator of stem cell biology and the KU team predicts that small ncRNA modifications will play a key part in future stem cell therapies.
“Most often, the onset of cancer appears linked to dysregulation in gene profiles,” explained Dr. Al Marzooqi. “There, the epitranscriptome of small ncRNAs is being directly implicated in the development of tumors by regulating the transcriptome of cancer stem cells. These small ncRNAs are being recognized for their potential to serve as biomarkers with diagnostic potential but the translation of these potential benefits to clinical applications is an ongoing effort. There’s no doubt that understanding how these small ncRNAs impact oncogenesis will help to identify how cancer develops and lead to more personalised approaches in its treatment.”
Jade Sterling
Science Writer
2 November 2020